The chemical reaction that takes place between cement and water is referred as hydration of cement. The reaction takes place between the active components of cement (C2S, C3S, C3A and C4AF) and water. The factors responsible for the physical properties of concrete are the extent of hydration of cement and the resultant microstructure of the hydrated cement.
On account of hydration, certain products are formed. These products are important because they have cementating or adhesive value. The quantity, quality, continuity, stability and the rate of formation of the hydration products are important.
The reactions of the compounds and their products are as follows:
C3S + H2O —> C-S-H + Ca(OH)2 + Heat
C2S + H2O —> C-S-H + Ca(OH)2 + Heat
Here, the C-S-H gel represents the calcium silicate hydrate also known as tobermorite gel which is the gel structure. The hydrated crystals are extremely small, fibrous, platey or tubular in shape varying from less than 2 mm to 10 mm or more. The C-S-H phase makes up 50-60% of the volume of solids in a completely hydrated Portland cement paste.
And, more quantity of Calcium Hydroxide is formed in hydration of C3S in comparison to C2S. The calcium hydroxide crystals liberated during silicate phase crystallizes in the available free space. The calcium hydroxide crystals also known as portlandite consists 20-25% volume of the solids in the hydrated paste.
Calcium Hydroxide is not a desirable product in the concrete mass because it is soluble in water and gets leached out, making the concrete structure porous, less durable, particularly in hydraulic structures. Therefore a cement with less C3S and more C2S is recommended for hydraulic structures.
Calcium hydroxide also reacts with sulphate present in soils and water to form calcium sulphate which further reacts with C3A and cause disintegration of concrete. This is known as sulphate attack.
The only advantage of calcium hydroxide is that it is alkaline in nature and it maintains pH value around 13 in the concrete which resist the corrosion of reinforcement.
Heat of hydration
The reaction of cement with water is exothermic. The reaction liberates a considerable quantity of heat. This liberation of heat is called heat of hydration.
The study and control of the heat of hydration becomes important in the mass concrete constructions like concrete dams. It has been observed that the temperature in the interior of large mass concrete is 50 degree Celsius above the original temperature of the concrete mass at the time of placing and this high temperature is found to persist for a prolonged period.
Different compounds hydrate at different rates and liberated different quantities of heat. Since retarders are added to control the flash setting properties of tricalcium aluminate (C3A), actually the early heat of hydration is mainly contributed from the hydration of tricalcium silicate (C3S). Fineness of cement also influences the rate of development of heat but not the total heat. The total quantity of heat generated in the complete hydration will depend upon relative quantities of the major compounds present in cement.
The development of strength of the four principle compounds of cement with age is as shown in figure below:
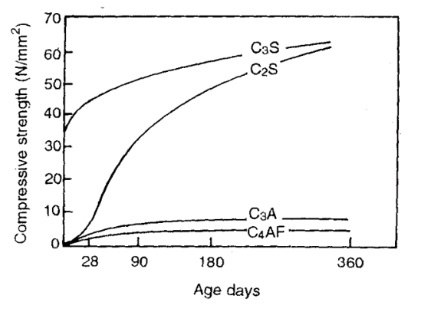
The rate of heat evolution of the compounds if equal amount of each is considered will be in the following descending order:
C3A > C3S > C4AF > C2S
The rate of hydration is increased by an increase in fineness of cement. However, total heat evolved is the same. The rate of hydration of the principle compounds is shown in figure below and will be in the following descending order:
C3AF > C3A > C3S > C2S
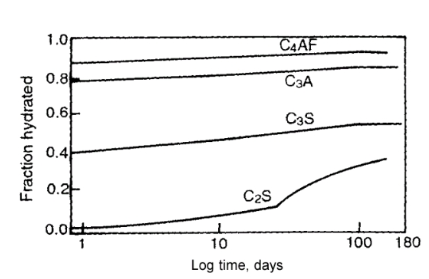
Quantity of Water Required in Hydration of Cement
It has been estimated that on an average 23% of water by weight of cement is required for complete hydration of Portland cement. This 23% of water chemically combines with cement compounds and therefore, it is called bound water.
A certain quantity of water is imbided with in the gel course, this water is known as gel water. It has been estimated that about 15% by weight of cement is required to fill up the gel course. Therefore, a total of 38% of water by weight of cement is required for the complete chemical reaction and occupy the space with in gel course.
